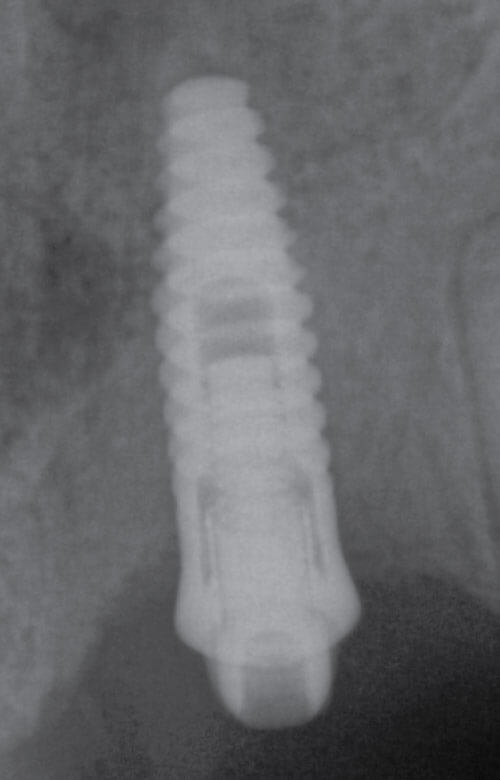
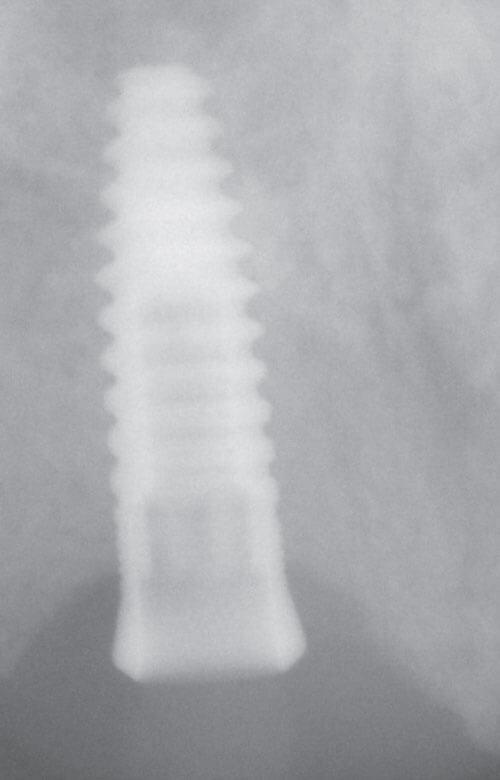
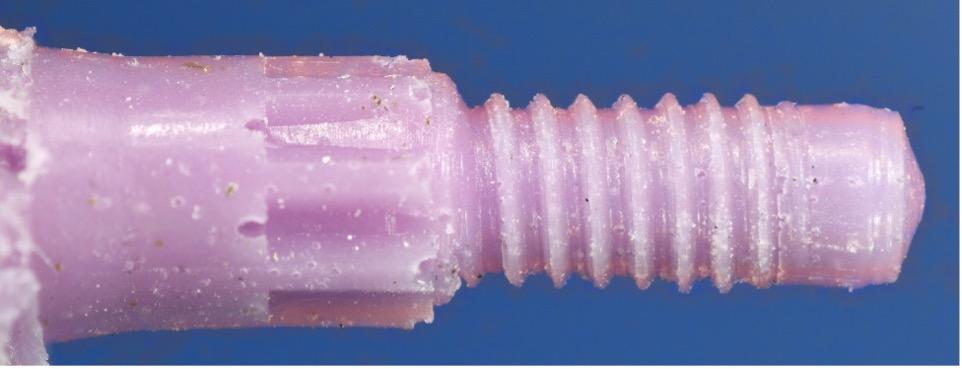
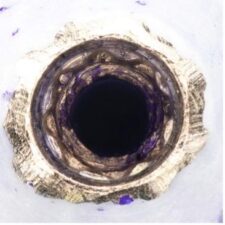
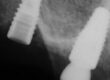
This case illustrates how difficult it can be to acquire a positive implant identification. The patient presented on referral for identification of the implant in the #8 site and recovery of a fractured abutment and retained abutment screw which had lost its drive geometry. The manufacturer of this implant was initially unknown as the dentist that placed the implant was no longer in practice and the treatment record was no longer available. For numerous reasons, this is not an uncommon occurrence. The referring Dr. had Spot Implant produce an identification report, but the list of 10 possible options proved to be of little to no help. All 10 had % scores greater than 50% with two having above 80%. It basically provided optional leads which had to be eliminated one by one. So, without a positive ID she was seen, and the fractured abutment and abutment screw were retrieved, fortunately without difficulty. With the internal aspects of the implant now available it was clearly evident this implant had a very unique interface. To further the identification process along, a polyvinyl impression of the internal geometry of the implant was obtained and the impression was plated with the Nickel Sulfamate process to produce a pure nickel metal analog of the implant. The following images are for your review.
Now with the specifics of the interface known, the options in the Spot Implant database narrowed significantly when sorting on an interface with an octa lobular indexing feature below a 7-degree (14-degree inclusive) conical connection. The outside diameter (OD) of the implant shoulder was physically measured with digital calipers intraorally, at roughly 4-4.1mm. The recovered abutment screw had a M1.8 x .35 thread. While external features often provide enough identifying data to positively identify the majority of implants, it wasn’t definitive in this case. The options narrowed to the 4.1 Biodenta implant. Unfortunately, an exhaustive search of the company found a website with US connections but with nothing active. Connections were attempted in Switzerland as well, as this was a Swiss company selling in the US, but again nothing proved successful when attempting to procure replacement components. Going forward, when deciding on an implant system, this should be another lesson when confronted with a plethora of new systems entering the world implant marketplace, all fighting for market share. It’s clear from the past that all of these companies, in their present form, will not survive the test of time. The clinical truth is many patients will eventually need long-term restorative maintenance care, as in this case, which often means having the availability to obtain quality replacement components.